
Therefore, special processes have been devised that, after 1950, changed titanium from a laboratory curiosity to an important commercially produced structural metal. Titanium cannot be obtained by the common method of reducing the oxide with carbon because a very stable carbide is readily produced, and, moreover, the metal is quite reactive toward oxygen and nitrogen at elevated temperatures. The preparation of pure titanium is difficult because of its reactivity. Hunter by reducing titanium tetrachloride (TiCl 4) with sodium in an airtight steel cylinder.
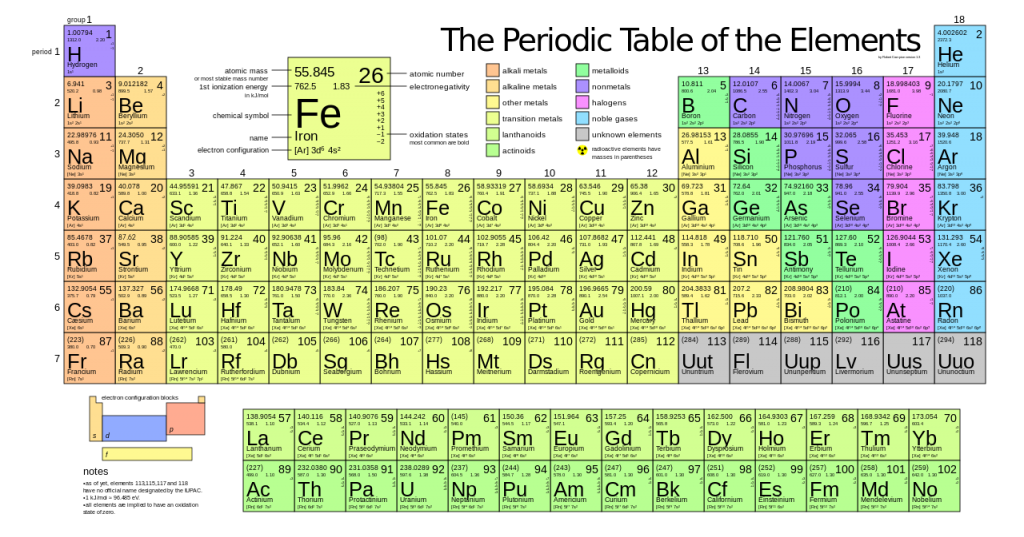
The metal was isolated in pure form (1910) by the metallurgist Matthew A. The two prime commercial minerals are ilmenite and rutile. It is also present in plants and animals, natural waters and deep-sea dredgings, and meteorites and stars. The metal is found combined in practically all rocks, sand, clay, and other soils. Titanium is widely distributed and constitutes 0.44 percent of Earth’s crust.
AS ELEMENT HOW TO
COVID-19 Portal While this global health crisis continues to evolve, it can be useful to look to past pandemics to better understand how to respond today.Student Portal Britannica is the ultimate student resource for key school subjects like history, government, literature, and more.This Time in History In these videos, find out what happened this month (or any month!) in history.
#WTFact Videos In #WTFact Britannica shares some of the most bizarre facts we can find.Demystified Videos In Demystified, Britannica has all the answers to your burning questions.Britannica Explains In these videos, Britannica explains a variety of topics and answers frequently asked questions.Britannica Classics Check out these retro videos from Encyclopedia Britannica’s archives.


 0 kommentar(er)
0 kommentar(er)
